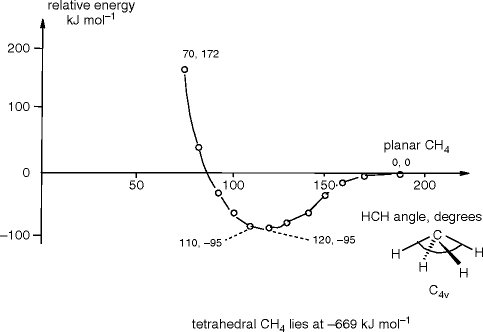
The answer cannot be found from this graph but rather an investigation of states of matter.
Smallest alkanes at room temp.
What is the smallest straight chain alkane that is a liquid at room temperature which is about 25 c.
Want to see the full answer.
These alkanes become gases at low temperatures due to the low number of carbon atoms in their structures.
Lower molecular weight alkanes tend to be gases and liquids while larger alkanes are solid at room temperature.
Properties and uses of alkanes.
The alkanes can exist as gases liquids or solids at room temperature.
Methane ethane propane and butane are all gases at room temperature.
Alkanes that have more than three carbon atoms form structural isomers.
Pentane through hexadecane are liquids.
What is the smallest straight chain alkane that is a liquid at room temperature which is about 25 c.
Asked jun 27 2020.
And pressure starting with pentane c5h12.
There is a drop in entropy when the alkanes change from gases to liquids at room temperature.
The unbranched alkanes methane ethane propane and butane are gases.
What is the physical state of the smallest.
Check out a.
The alkanes are liquids at room temp.
Methane ethane propane and butane are the alkanes that are gases at room temperature.
The homologues larger than hexadecane are solids.
Alkanes tend to make good fuels.
Pentane and the seven others displayed in this graph are liquids.